Breast cancer researchers at UCLA saved thousands of lives by developing groundbreaking treatments and establishing a new paradigm in cancer research.
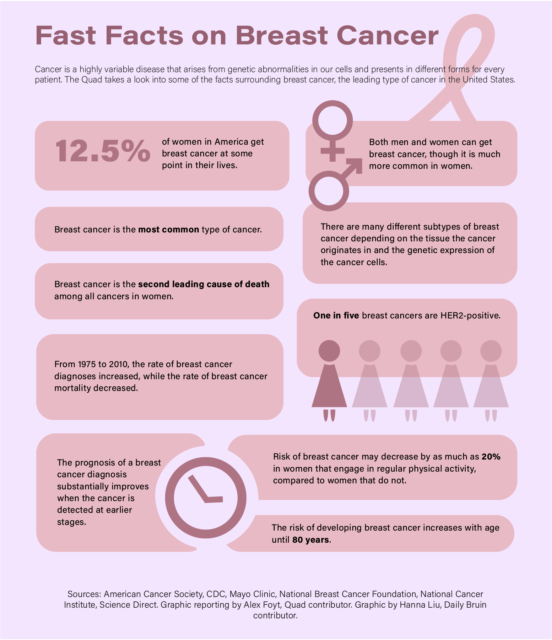
According to the National Cancer Institute, cancerous cells result from genetic mutations to any of the body’s normally functioning cells, which cause these cells to grow and replicate continuously.
Dr. Richard S. Finn, a professor of Medicine at the UCLA Geffen School of Medicine and joint director of the Signal Transduction and Therapeutics Program at the Jonsson Comprehensive Cancer Center at UCLA, said that cancer has historically been treated primarily through chemotherapy.
“(Chemotherapy treatments) are just chemicals,” Finn said. “Poisonous chemicals … that kill cancer cells. They kill normal cells, which is why they’re so toxic.”
Now, with the development of molecular therapeutics from researchers like Finn and his colleagues, non-discriminating toxic treatments like chemotherapy are no longer the only options available to cancer patients.
“The real paradigm shift that differentiates how we practice medical oncology today versus even 20, 30 years ago is the impact of molecular therapeutics or drugs that are focused on tumor biology,” Finn said.
Dr. Dennis Slamon, director of Clinical and Translational Research at UCLA Health, helped pioneer molecular therapeutics by developing the breast cancer drug Herceptin, which he said has been administered to over three and a half million women and saved thousands of lives.
Slamon said Herceptin was created to target the pathology of a specific variety of breast cancer cases called HER-2-positive breast cancer, which originates from a non-heritable mutation in a gene that regulates the normal growth of cells in breast tissue.
According to Slamon, the HER-2 gene codes for a type of protein called a growth factor receptor, which sits on the surface of cells like antennas and transmits growth signals from the external environment to the nucleus of the cell.
Slamon added that all normal human cells have a single copy of the HER-2 gene, which is expressed by breast cells to produce up to 50,000 copies of the HER-2 protein that occupy the cell membrane.
However, in HER-2-positive cases, Slamon said the machinery that copies DNA during cellular replication gets stuck on the HER-2 gene and produces extra copies, thus producing more HER-2 proteins as a result.
“When this abnormality happens, that can go up to more than a million, frequently two or three million. So it’s a two log increase of this protein on the cell,” Slamon said.
Slamon added that more copies of the growth factor receptor on the surface of the cell make it hypersensitive to environmental growth factor signals and therefore grow aggressively, forming a tumor.
Slamon said that once him and his team characterized the HER-2 protein, they set about finding a way to target it with therapeutics. While the portion of the protein on the interior side of the cellular membrane was inaccessible, they discovered they could target the exterior portion with antibodies.
According to the National Human Genome Research Institute, antibodies are proteins derived from one’s immune system that can recognize and bind to specific identifying structures on cell surfaces.
Slamon said his discovery that antibodies could be used to target HER-2 proteins led to the creation of Herceptin, an FDA-approved antibody for humans that attacks HER-2-positive cells on two fronts.
“That antibody covers that antenna and can mess up the signaling process so that it doesn’t signal the growth signal,” Slamon said. “In addition, if there’s enough of it on the cell, there’s an overabundance of the antibody attaching to it. It lights up that cell for the immune system, particular cells in the immune system, that come in and attack the cell.”
Finn said Herceptin not only saved thousands of lives but also directed the field of cancer research towards developing further targeted therapies.
“What we did was prove that there is such a thing as targeted cancer therapy, as opposed to throwing in a nonspecific bomb and hoping to kill more bad cells than good cells,” Slamon said. “We could find out what’s broken in the cell, in the cancerous cell, and see if we could devise a therapy that targets that specifically.”
Eric Bilotta, a UCLA alumnus currently studying drug resistance in cancer cells at Scripps Research in San Diego, said targeted cancer therapies are the future of cancer research.
“You do chemotherapy which just targets any fast-growing cells. … There’s so many secondary issues that arise with chemotherapy,” Bilotta said. “But if you can do a very site-directed treatment that will target cells in a very direct manner, then that is how you can target cancer and kill it.”
Bilotta said future cancer treatments must not only be specific to the type of cancer that a patient might have but specific to the patients themselves. In some cases, a drug that kills the cancer of one patient may cause increased growth in another patient.
“There’s some hospitals and some private companies that now will take samples of … your cancer and your tumor, and will do a high throughput screening of a huge drug library to see what affects it the most,” Bilotta said. “It’s very patient-specific. It’s really important because it’s not a one-size-fits-all.”
With the wide variety of developing cancer treatments, cancer patients can expect better outcomes with more tailored cures and fewer negative side effects.
“(It’s an) incredibly exciting time. There’s a ton to do. There are more questions than answers right now. And all good questions, but there are better tools to ask those questions,” Slamon said. “So it’s a rewarding time to be doing this work. The field is really broken wide open.”